Due to their importance, salts have already been mentioned several times in previous sections. We mentioned them when we looked at the key differences between sweet and saline water wetting. We discussed the role of salts as a moisture barrier because they significantly affect the drying and breathability of the wall fabric. Salts also make the masonry electrically conductive subjecting the wall fabric to a wide array of electrical effects.
in this section we are going to summarize the most important things about salts and how they affect old buildings. Understanding these effects is important because salts significantly affect the wall fabric and old walls with high salinity must be renovated differently from walls with low to moderate salinity.
What are Salts?
Salts are minerals consisting of two electro-chemical parts called ions. The electrically positive part (in chemistry called "cation") is normally a metal, the most common ones being: Na, Ca, K and Mg. The electrically negative part (anion) are other chemical groups, most commonly chlorides (Cl), nitrates (NO3) and sulphates (SO4).
These 7 ions - the 4 metals (Na, Ca, K, Mg) and 3 other groups (chlorides, sulphates, nitrates) - in combination produce 4 x 3 = 12 elementary salts, which account for over 98% of the salts in old walls. In reality there are more than 12 types of salts but these are the most important ones.
Table salt (NaCl) is just one of the many existing salts.
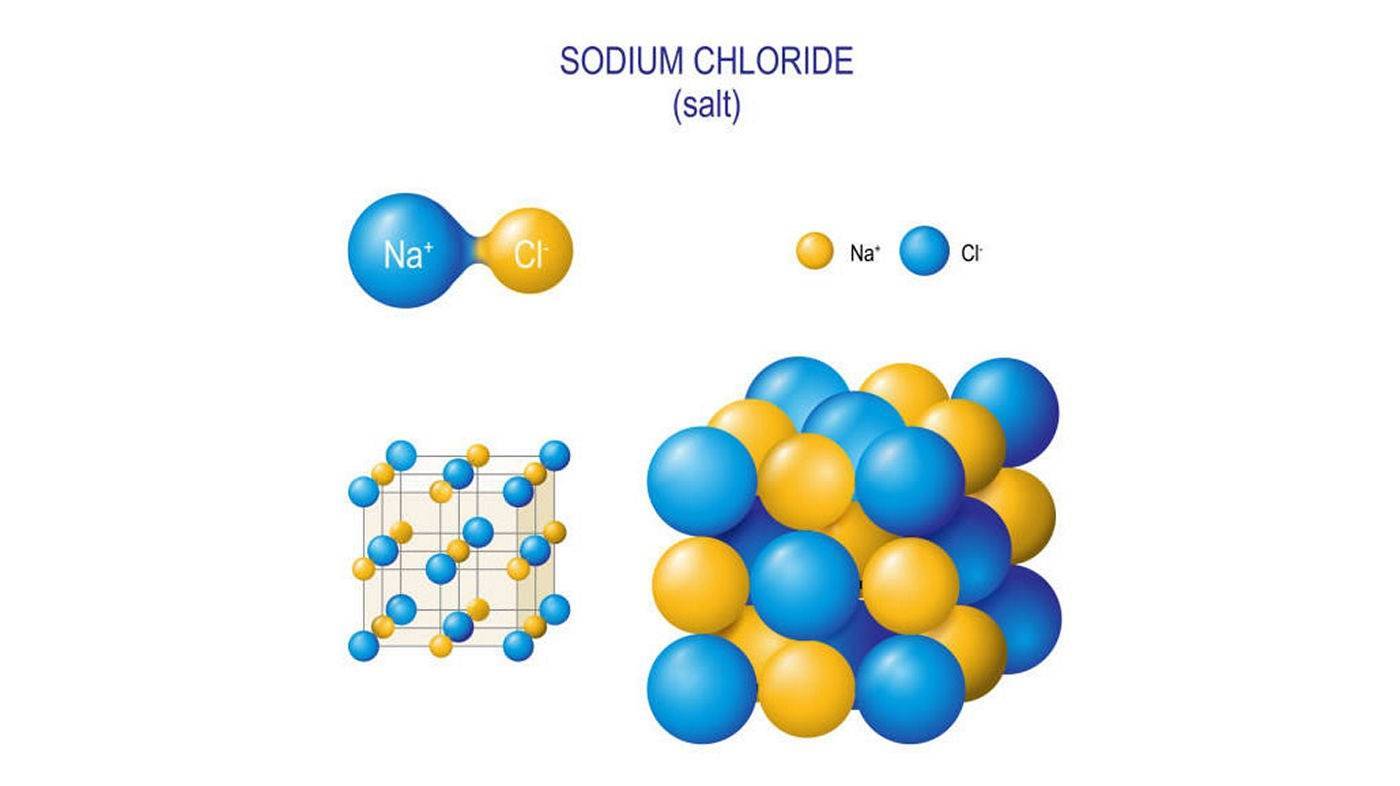
Salts in dry state have an organized crystalline structure
In dry state, the salts are organized in a 3D-lattice, the solid salt crystals being held together by intermolecular electrical attraction forces. Small crystals are white or yellowish powders and can have a salty or bitter taste.
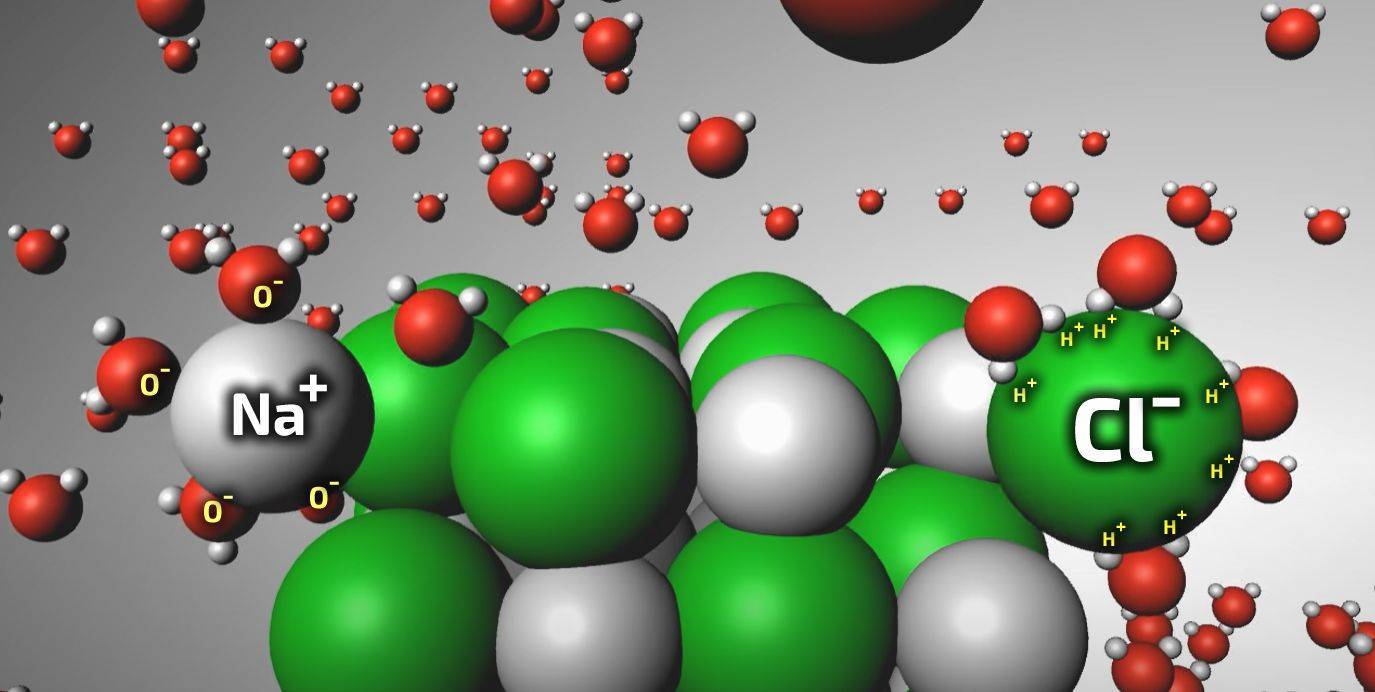
In the presence of water, however things change. Salts become diluted by water: the charged salt lattice gets surrounded by charged water ions (positive H+ and and negative OH- ions) pulling the salt lattice apart, breaking down its organized crystalline structure. The positive and negative salt ions become separated, travelling independently with the liquid water into the masonry, but retaining their individual positive or negative charges in the solution.
Once humidity from the masonry evaporates, salts re-crystallize - either as the same salt or a new one, forming one of the 12 common salts.
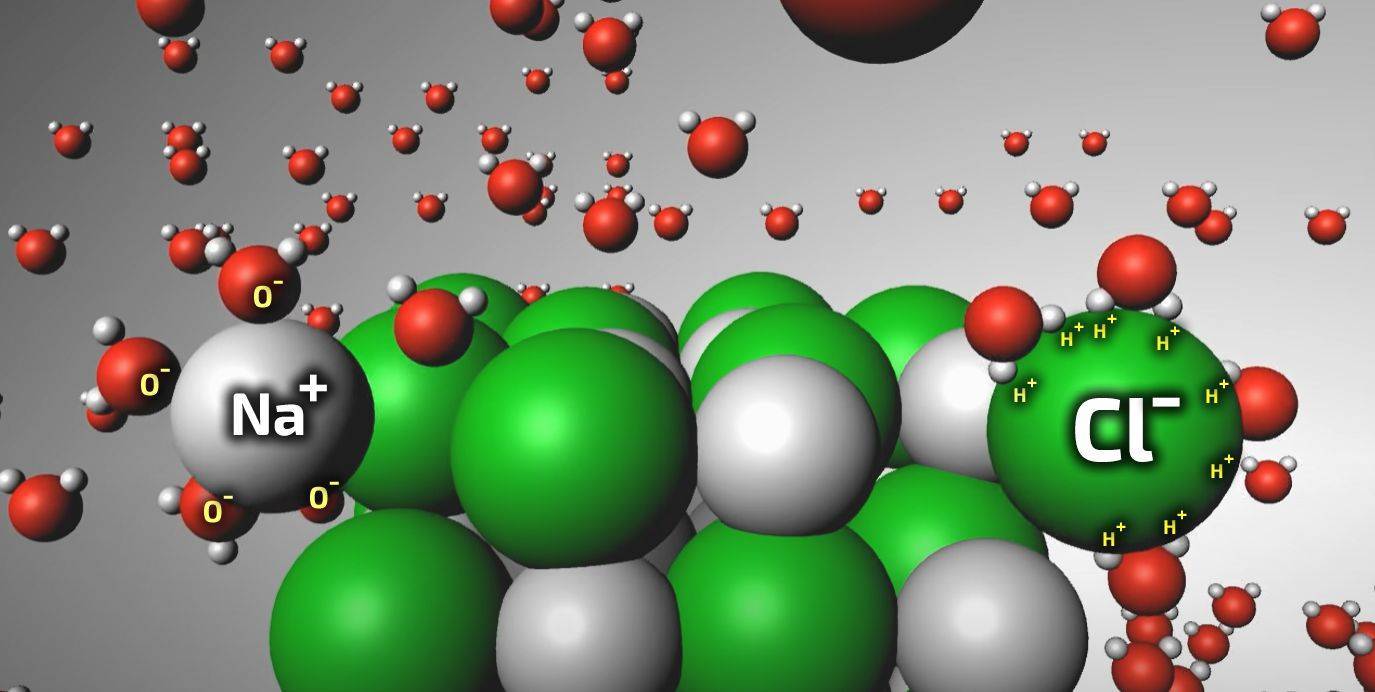
Salts being dissolved by water
Where Salts Originate From?
In small quantities, salts are natively present in bricks. Bricks, made of clayey soil, naturally contain a small quantity of salts. However, most salts are deposited into the building fabric from various sources, gradually "polluting" the building fabric over time.
Here are the most common sources of salts:
- The ground: near ground level the primary mechanism that drives salts into the building fabric is rising damp. The soil contains many salts (minerals) in diluted form. These salts are carried up into the wall fabric through various mechanisms, resulting in high quantities of salts in the lower part of the building fabric. White powdery salt crystallization in the lower meter or so near ground is a common occurrence in old buildings.
- The air: buildings close to the sea are subject to sea salts by the wind, mist and fog. These salts are washed-in into the building fabric by rainwater, accumulating over time,
In large cities air pollution (sulphur gases) combined with rain creates acid rain, which reacting with the lime creates salts, leading to the crumbing and decay of stones. - Chimneys: interestingly enough, chimneys are very much subject to the erosive action of salts. The black soot in chimneys is very rich in salts. These salts over decades and centuries permeate the chimney walls, resulting in the discoloration, crumbing and general decay of chimney walls or ceiling areas. Many "mystery" dampness problems, when no water ingress is present, are caused by salts.
- From modern cement: due to the very high firing temperatures (around 1500 °C) used during the production of cement plasters, many chemical reactions that take place in cement are undesirable, negatively affecting the quality of cement. To mitigate them, various additives are mixed into the cement during the manufacturing process. For e.g. to make cement more workable, gypsum salts (CaSO4) are added into the mix. These salt additives end up in the old wall fabric resulting in irreversible damage of the historic building fabric.
Most Common Type of Salts
The most common types of salts found in the fabric of old buildings are:
Chlorides (Cl)
Chlorides are plain sea salts (e.g. common table salt). Sea salts are transported by wind and fog inland ending up in the ground or washed into the buildings.
Salts sprinkled onto the roads during winter are also chlorides that end up in the ground then absorbed into the building fabric.
Nitrates (NO3)
Nitrates are salts predominantly originating from the decomposition of waste matter. They are prevalent in farming areas. Animal- and farming byproducts such as manure or fertilizers contain high quantities of nitrates, which have been affecting that old farm building or barn walls for centuries. When these buildings are converted to dwellings the very high content of the walls must be known and mitigated.
Churches, chapels, graveyards and former battle grounds also contain a large amount of nitrates.
Sulphates (SO4)
Sulphates are one of the most dangerous or damaging type of salts as they crystallize in long needle-shape crystals which can cause a lot of damage to the building fabric.
Sulphate salts can be found in modern cement, added to it as an additive to make cement set slower. Under certain conditions the sulphates in cement can react with other salts in the environment, resulting in extreme salt expansion known as sulphate attack, causing severe damage to concrete structures and plasters.
Sulphates are also formed after combustion, being abundantly present in the chimney soot or in emission gases of the polluted atmosphere. When airborne sulphates are washed-down by rainwater, the resulting acid rain containing weak sulphuric acid, dissolves the lime, damaging old limestone statues or façades.
The presence of these salts can be measured and assessed with specialist tools. We do this on a regular basis as part of our professional building surveys.
The Effect of Salts – How Salts Damage old Buildings?
Here is a video explaining and demonstrating some of the damages salts can do on old buildings.
Salts affect old masonry in many ways, here are the most important mechanisms:
1. Salts Absorb Humidity (Hygroscopic Action)
Every time when ambient humidity increases or the temperature decreases for whatever reason (e.g. wet season, period of rain, day-night variations etc.) salts tend to liquefy. Salts have a very interesting property: they are "hygroscopic" which means they can absorb moisture from the ambient air.
One salt molecule in a humid environment can attract and hold many water molecules, making the wall surface look damp. A well-known example of this is the behaviour of common table salt which becomes less crisp or soft in a high-humidity environment by attracting moisture from the surrounding air.
As a result salty bricks and plasters retain a lot more humidity than non-salty building materials. For e.g. in a lab test experiment while a non-salty brick absorbed from the air 31 grams of vapours (blue), a salty brick, under the exact same conditions, absorbed 141 grams of water vapours (green) - 4.5 times more than the non-salty masonry.
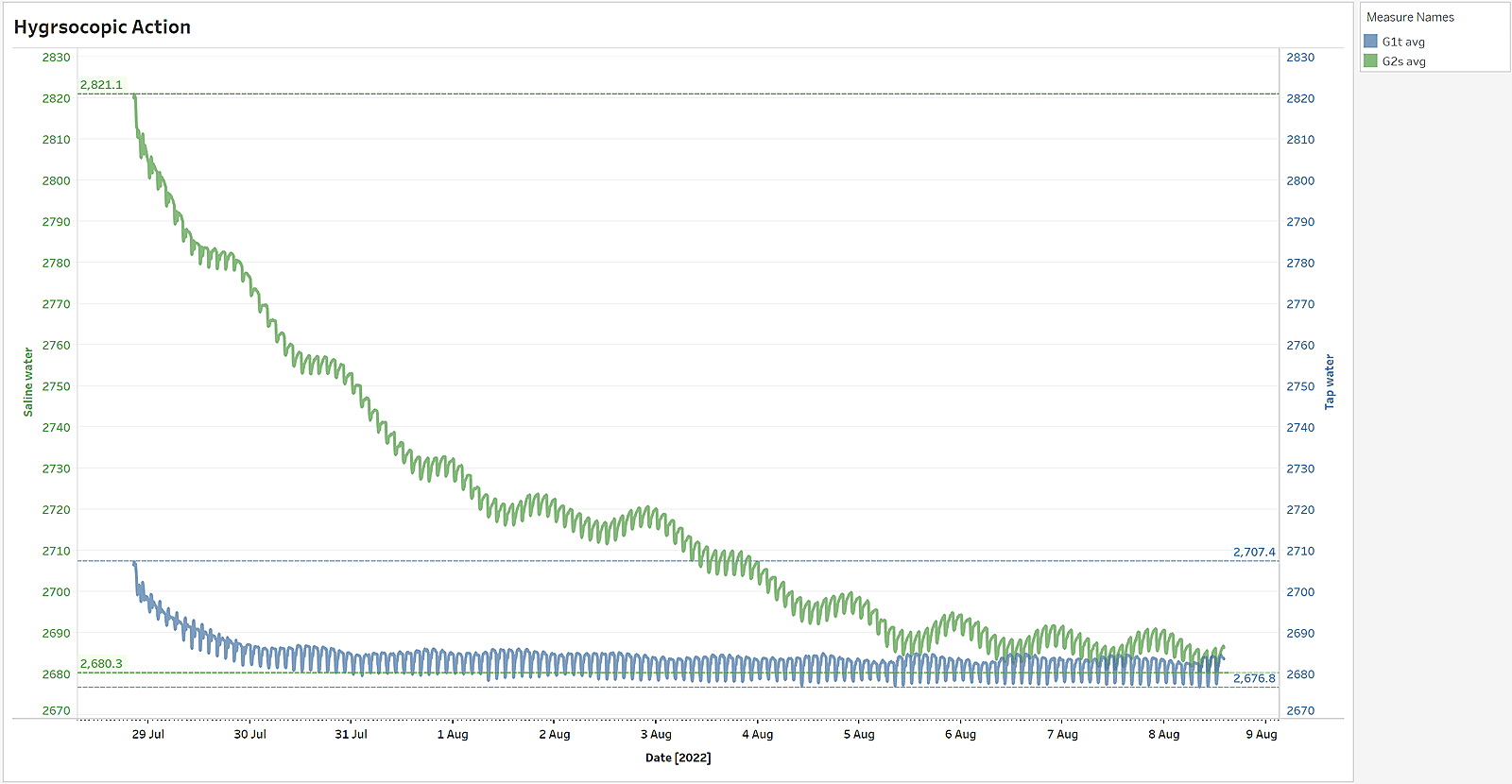
Salty bricks retains a lot more water than non-salty bricks
As most salts in an old wall, due to evaporation, get deposited on the surface (known as efflorescence) or just under the surface (subflorescence), in a high-humidity environment salts can make a wall fabric look damp even if the wall in depth is significantly drier.
This brings up an important technical point about the re-plastering of old, salty walls. The old, salt-laden plaster MUST be removed completely and affected wall sections must be fully re-plastered in order to ensure a salt-free = dry surface.
Retaining an old salt-laden plaster and just skimming it over in order to save money is a common and often costly renovation mistake which one can easily avoid by understanding what salts do and how they behave. A salt-resistant lime base coat must also be used to protect the longevity of the plastering.
2. Salts Act as a Moisture Barrier, Blocking Evaporation
Just as salts absorb humidity from the environment, they also won’t release it easily when ambient drops or when the wall starts drying out. In comparison to freshwater wetting, it takes a lot more energy to make water evaporate from a salty wall fabric.
In our lab experiment a non-salty brick it took 2 days to reach a new state of equilibrium, while a salty brick it took 8 days (4 times longer) to reach the equilibrium. This can be seen on the above graph, where the "flattening" time of the two lines is very different: it took moisture (blue) 2 days to evaporate out from a non-salty brick, and 8 days for the moisture (green) to evaporate out from a salty brick and stabilize.
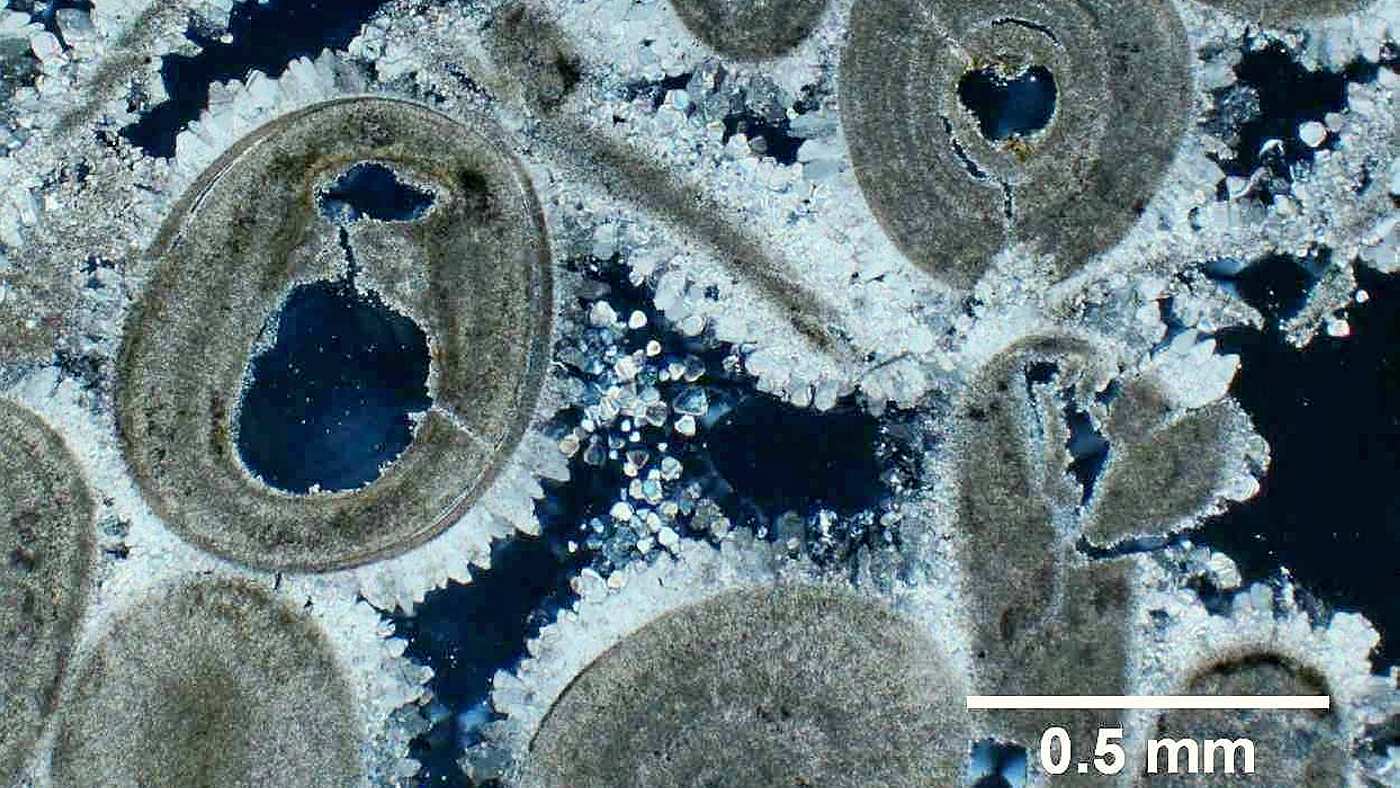
3. Salt Crystallization is a Leading Cause of Fabric Damage
When humidity levels decrease or the temperature increases, salts crystallize. During crystallization salts expand in volume by 5 - 10 times (500% to 1,000%). The expansion generates enormous forces inside the masonry (up to 800 atmospheres), capable of breaking down even concrete. The high intermolecular pressures result in the crumbling and breakdown of paint, plaster and historic wall fabric, leading to crumbling, spalling, flaking, cracking or peeling of the building fabric.
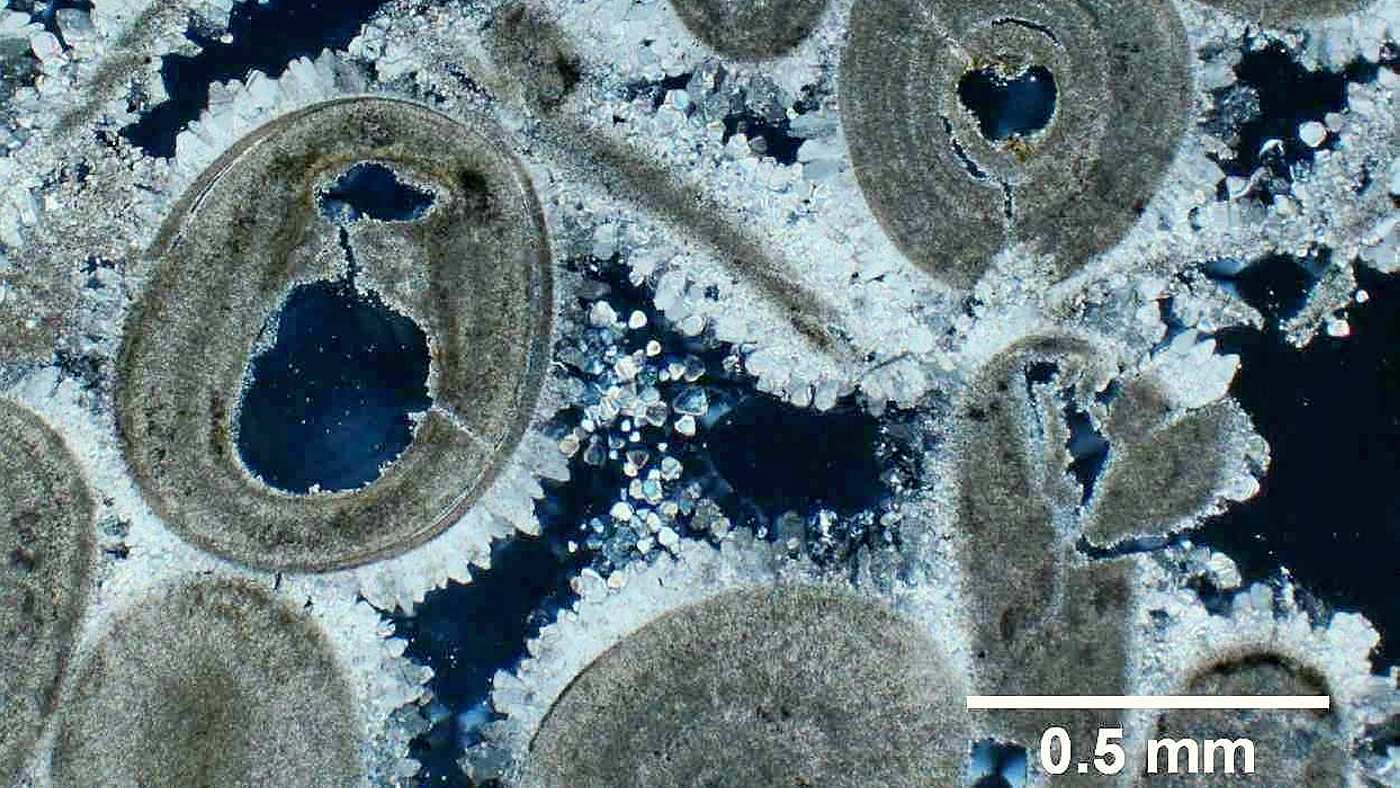
Salt crystallization in mortar under the microscope, leading to cracks in the fabric
Salt crystallization is similar to water freezing into ice. Ice crystals "only" expands in volume by about 10% yet the induced crystallization pressure can crack iron or steel pipes. In comparison, salts expand many times, thus capable of creating significant damages.
Salts are the primary reason behind the irreversible damage and loss of historic building fabric. They are the main reason behind the damage and breakdown of plasters and why old buildings need to undergo a major replastering cycle every few years.
4. Salts Make the Wall Fabric Electrically Conductive
There is another important aspect of wet salts: they are electrolytes that make the wall fabric electrically conductive. A non-salty wall fabric is an insulator. A salty wall-fabric becomes a good conductor.
As we can see below, the electrical resistance of a non-salty wall fabric (red) increases very steeply as humidity starts to evaporate. A salty wall fabric, on the other hand, stays conductive for a very long-time (grey), even after the fabric dries out. The conductivity of a salty wall fabric is about 100 - 10,000 times better than of a non-salty wall fabric.
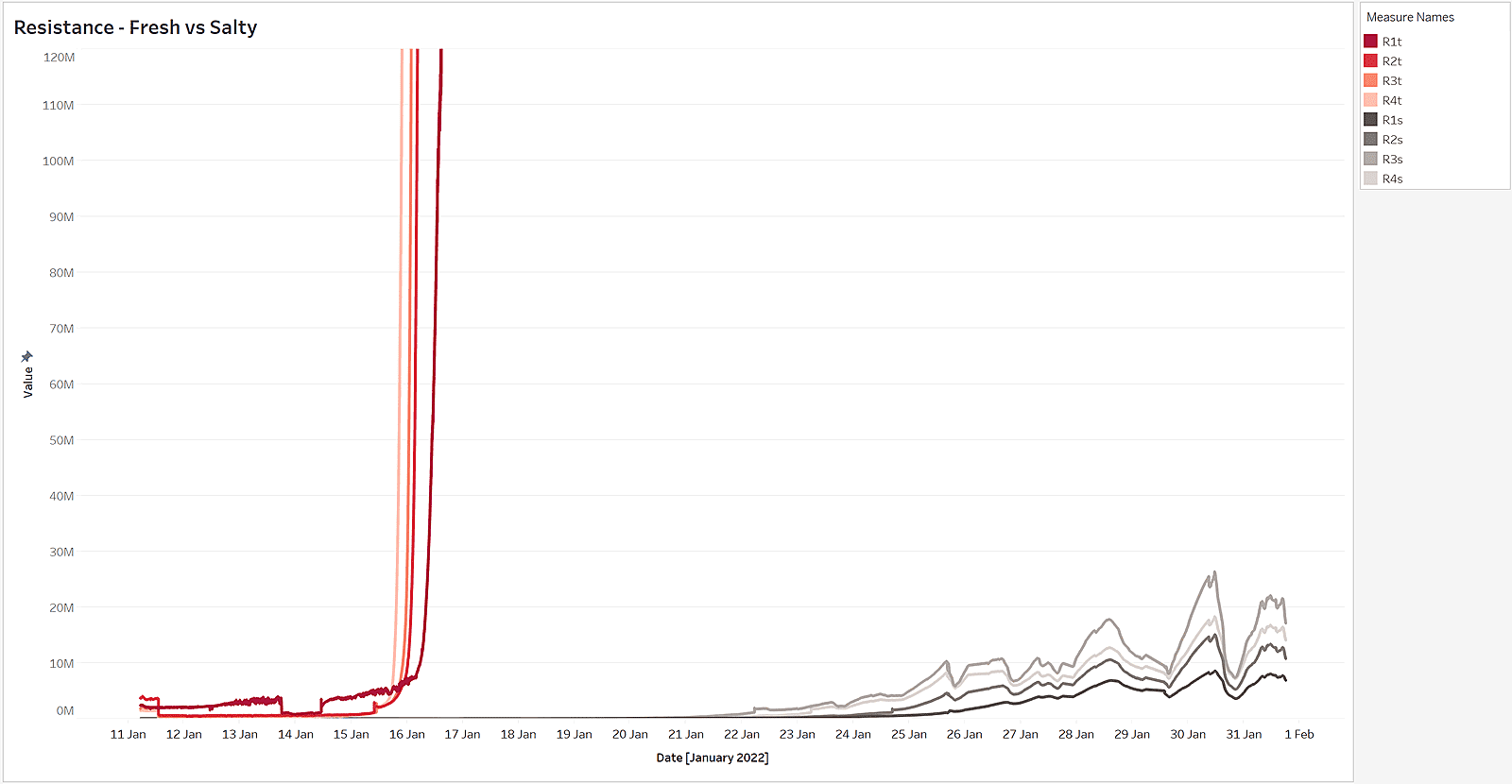
Electrical resistance of salty and non-salty walls differs significantly
Moreover, in any conductor, surrounding electromagnetic fields induce electrical voltages and currents. In the wall fabric this translates to the presence of small wall voltages and currents that interfere with the movement of water molecules and salt ions inside the wall fabric.
In other words, by making the wall fabric electrically conductive, salts link the wall fabric to the effects of the surrounding electromagnetic environment, making the walls subject to a multitude of electrical phenomena driven by the surrounding electromagnetic environment.
Electrical phenomena in damp masonry are discussed in much more detail here,
Solutions, Remedies, Best Practices
As a building ages, more and more salts accumulate inside the building fabric, leading to higher and higher salt concentrations. The salts become part and parcel of the building fabric and can't be easily removed from it.
Here are some real-life buildings with various salt damages:




































One of the most common mistakes during the renovation of old buildings - especially during farm or barn conversions - is not being aware of the potential problems salts can create. Some common problems found in old farms and barns are highlighted by the video below.
Various desalination procedures have been developed, but these complex, difficult to apply and require professional intervention. Because there is no easy desalination procedure, most salt problems are managed by using the right type of plasters.
Replastering an old salty wall with a breathable lime plaster is not a long-term solution. In the presence of high salinity, the fresh lime plaster will be short lives, it will be broken down by the crystallizing salts found in abundance in old farm buildings.
The right solution is using special salt-resistant lime plasters. The technology of these plasters originates from ancient Rome. The Romans figured out that if the soft lime is mixed with volcanic sands and ashes from Mount Vesuvius it will result in salt-resistant lime mixes that can even withstand sea water for many decades. These pozzolanic lime plasters have been used in Venice for centuries, performing extremely well in damp and harsh environment.
Applying a salt-resistant lime base coat under the main lime coat will extend the life expectancy of your plaster by about 10X, making your lime plaster last much-much longer, without sacrificing breathability in any way.
Feel free to reach out with any questions.
Research Data – Scientific Papers on How Salts Affect Old Buildings
The destructive effect of salts onto old buildings is of great concern and as such it is researched by many universities worldwide. For those interested in the research data, here are some international research papers about the importance of salts in building conservation, the salt crystallization process and their effects onto old masonry.
The role of sea salts in the occurrence of different damage mechanisms and decay patterns on brick masonry – Construction and Building Materials, March 2004
Investigating the effects of humidity and salt crystallisation on medieval masonry – Building and Environment, November 2000
Impact of salt crystallization on the mechanical properties of structural masonry: An experimental and numerical study – Construction and Building Materials, July 2022
Experimental investigation on bricks from historical Venetian buildings subjected to moisture and salt crystallization – Engineering Failure Analysis, October 2014
Effect of salt crystallization on stones of historical buildings and monuments, Konya, Central Turkey – Building and Environment, 2007
Characteristics and deterioration mechanisms in coral stones used in a historical monument in a saline environment – Construction and Building Materials, January 2020
Experimental research on hygroscopic behaviour of porous specimens contaminated with salts – Construction and Building Materials, June 2004
Do environmental conditions determine whether salt driven decay leads to powdering or flaking in historic Reigate Stone masonry at the Tower of London? – Engineering Geology, April 2022
Research into the influence of subsoil on sulphates, nitrates and chlorides accumulated in renovation plasters used for rehabilitation of monuments in the Czech Republic – Journal of Cultural Heritage, February 2021
Study of nitrate contaminated samples from a historic building with the hygroscopic moisture content method: Contribution of laboratory data to interpret results practical significance – Journal of Cultural Heritage, March–April 2018
Electrokinetic desalination of a farmhouse applying a proton pump approach. First in situ experience – Construction and Building Materials, February 2020
Electrokinetic removal of Ca(NO3)2 from bricks to avoid salt-induced decay – Electrochimica Acta, March 2006
The short-term effects of mortar joints on salt movement in stone – Atmospheric Environment, May 1997
Influence of paints on drying and salt distribution processes in porous building materials – Construction and Building Materials, May 2009
Simulation-based analysis of the differences in the removal rate of chlorides, nitrates and sulfates by electrokinetic desalination treatments – Electrochimica Acta, February 2013
3D-resistivity imaging and distribution of water soluble salts in Portuguese Renaissance stone bas-reliefs – Engineering Geology, July 2012
Computational fluid dynamics (CFD) modeling of microclimate for salts crystallization control and artworks conservation – Journal of Cultural Heritage, July–August 2014