In the previous sections we have looked at some of the molecular phenomena behind rising damp - the electrical double layer and the effect of short-range forces acting inside capillaries - as well as at some external drivers such as heating.
In this section we are going to put all pieces together, describing the overall mechanism of rising damp and the phenomena that contributes to its formation.
Water Transport Mechanisms in Masonry
Rising damp is a combination of VAPOR and LIQUID transport mechanisms as vapor and liquid phases jointly co-exist inside the capillaries.
The wetting process of a dry building material is a gradual process. It starts with vapor diffusion and adsorption, and leads through capillary condensation to capillary flow - unsaturated to saturated - as shown below:
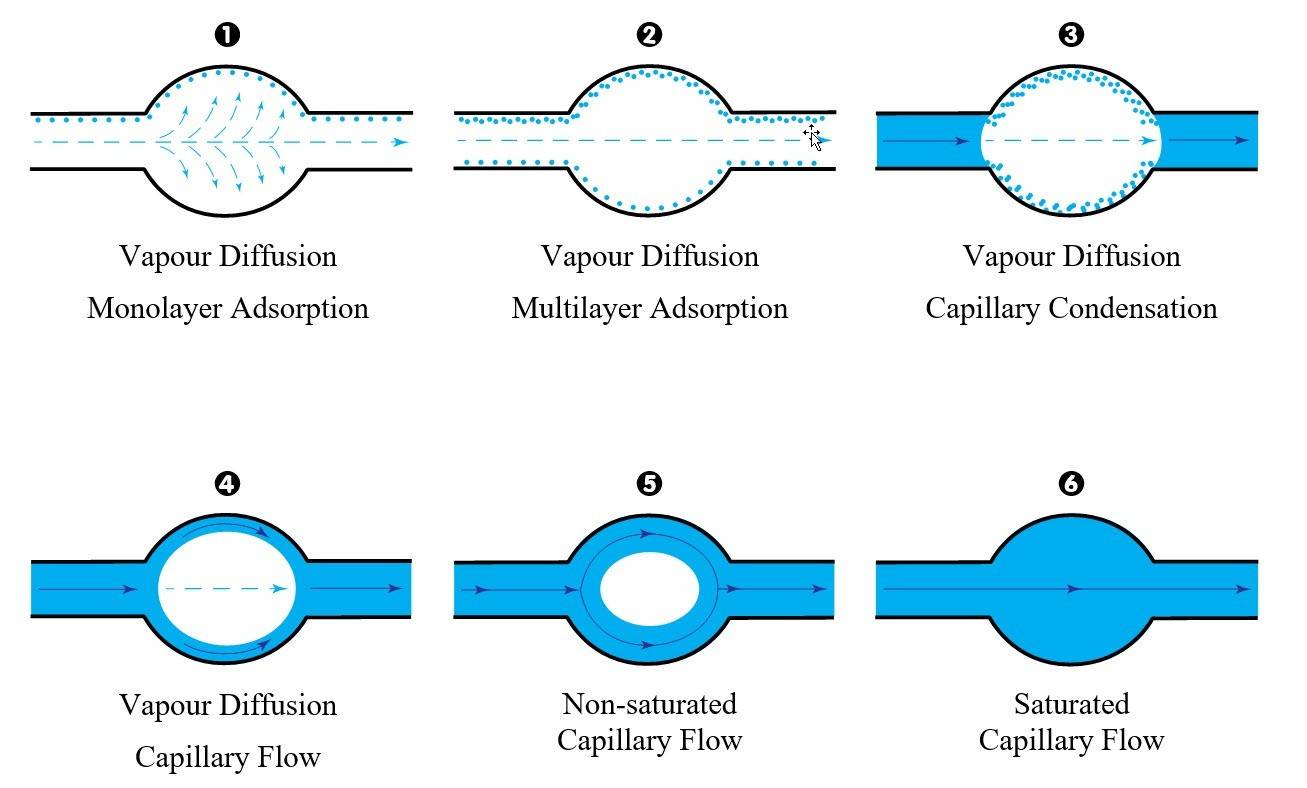
Here is a brief explanation of these scientific terms:
Vapour diffusion
(vapor)
The passive movement of vapour molecules between from high to low concentration (natural spreading) until equilibrium is reached.
E.g. perfume or smoke spreading in a room
Adsorption
(vapor)
The attachment of atoms (e.g. water) onto a solid surface, creating an extremely thin film, initially one then several molecules thick. (monolayer and multilayer adsorption).
E.g. misty window
Capillary condensation
(vapor > liquid)
The occurrence of liquid water and wetting of solid surfaces, the filling of pore spaces with water, A change of phase occurs from gas to liquid.
Capillary flow
(liquid)
The flow of liquid water inside the capillaries.
How Rising Damp Starts
The wetting process of a dry wall, in the earliest phase, is triggered by temperature differences. The base of the walls is colder while upper areas are warmer, due to the cooling effect of the ground and the presence of heating. Because warm air is lighter and cool air is denser, the temperature differences translate to pressure differences - higher pressure at the cold base and lower pressure towards the top of the wall.
These pressure differences create a pumping mechanism, moving the vapors up inside the initially dry capillaries, where the deposition of water begins, initially in the form of a very thin, microscopic film.
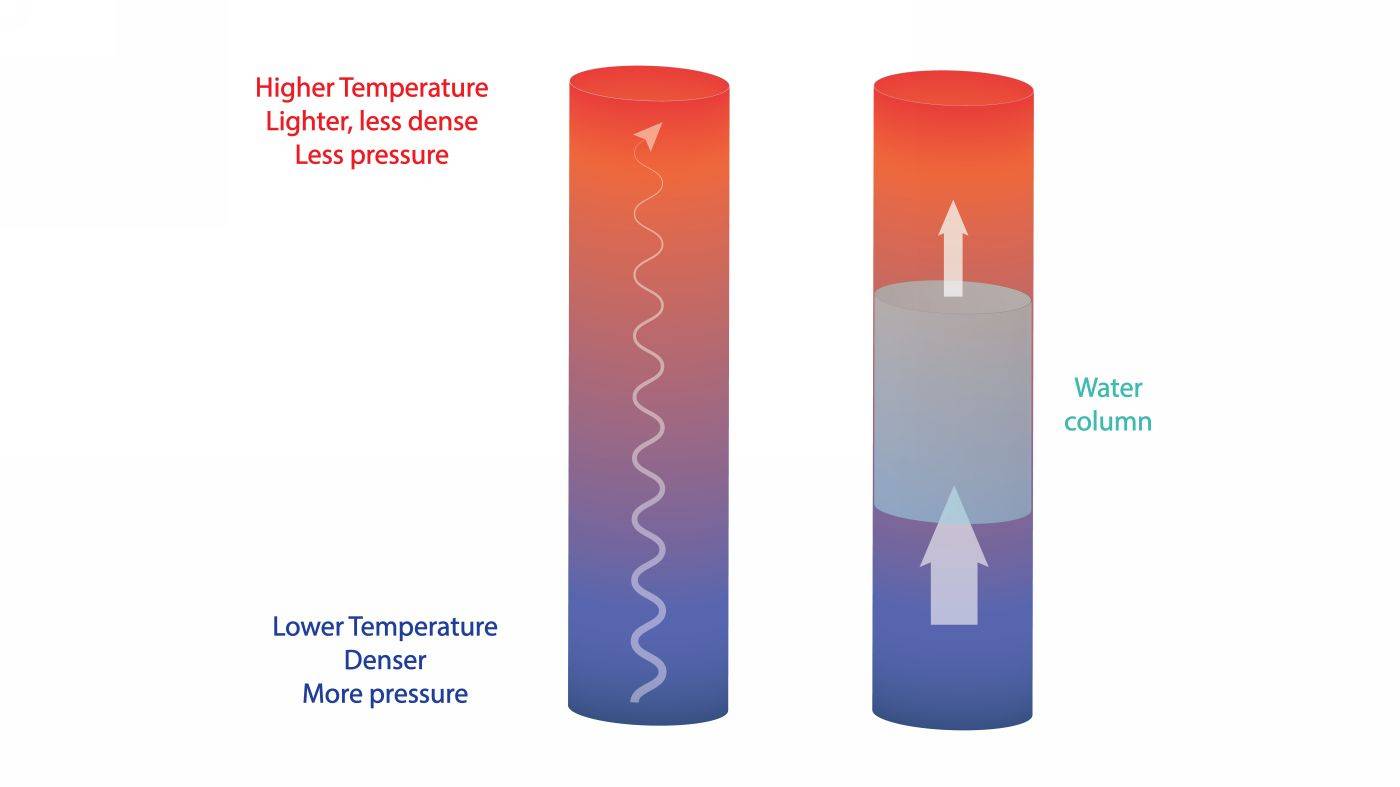
Temperature or pressure differences move the water upwards inside the wall capillaries
Liquid Films & Salt Transport in Nanopores
Small, narrow capillaries can get saturated very easily, leading to the build-up of liquid films along the capillary walls.
In very narrow capillaries (with diameters less than 200 nm = 0.2 microns) where capillary water flow is not possible due to the surface tension of water, salt transport can still commence through liquid films on the surface, even in the absence of a capillary flow. This rising damp mechanism, the formation of liquid films in extremely narrow capillaries (2-50 nm) has been studied since the mid 1950s.
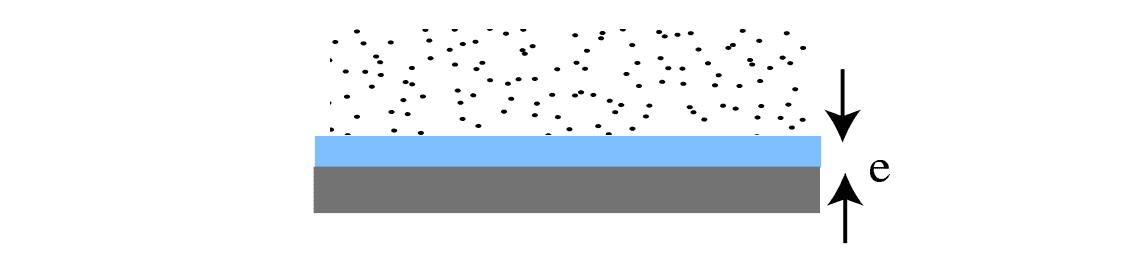
Very narrow capillaries where water flow is technically not possible can still develop liquid films
Capillary Flow - Electrokinetic Phenomena
Once capillary flow has been established, new rising damp mechanisms - the electrokinetic phenomena - enter into play and the process gets significantly more complex.
Electrokinetic phenomena (electro = electric, kinetic = motion) describe a number of short-range molecular phenomena that occur at any solid - liquid interface (e.g. capillary - water boundary), dealing with the movement of water and / or particles suspended in water (e.g. salts). These include some known phenomena such as osmosis or electroosmosis, but there are a lot more - the most important ones are summarized in the table below:
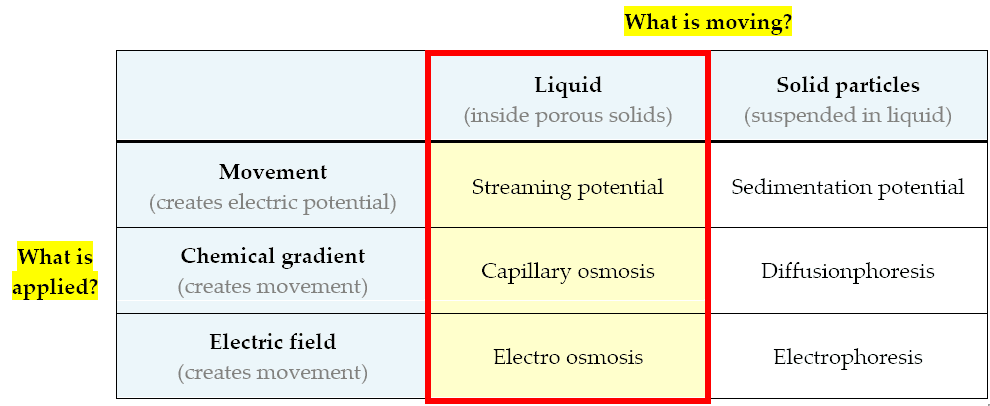
Electrokinetic phenomena contributing to the presence of rising damp
These forces play a significant role at the wall-water interface also known as the electrical double layer. The negative wall surface attracts the positive end of the water molecules, bonding them to the capillary surface by electrostatic attraction.
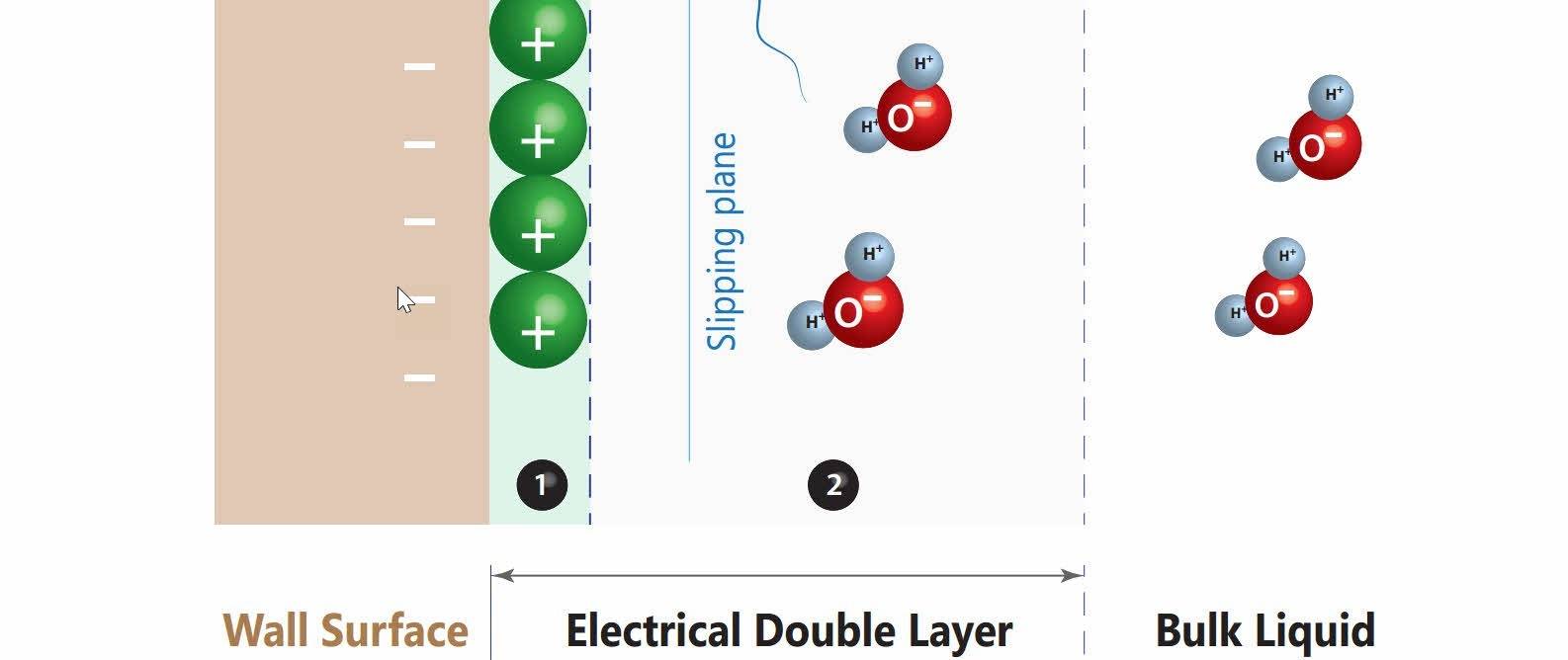
The electrical double layer "bonds" the water to the surface of the capillaries through electrostatic forces
Small local charges (e.g. crystallized salts) as well as external electric and magnetic fields can also significantly influence the forces in the electrical double layer, especially alternating-current (AC) or pulsed signals, the effects of which - due to their complexity - are not fully understood.
The Presence of Ground Salts
Liquid moisture transport draws up various diluted salts from the ground (chlorides, nitrates and sulphates) which over time accumulate into the building fabric.
A high concentration of salts can often be found in the upper area of the building fabric (about 1 meter high) - this concentration difference turns on another lifting force: osmosis - water starts migrating towards high concentration salt areas,

The presence of salts, through crystallization, can damage the wall fabric significantly; while the effect of hygroscopic moisture onto the brickwork is also not negligible.
Summary
The bricks, mortar, water and salts form a complex electrical-physical-chemical system subject to many short-range molecular forces.
This makes rising damp a very complex phenomenon, with many vapour and liquid transport phenomena contributing to the rising damp mechanism, including:
- Vapour diffusion (vapor)
- Monolayer adsorption (vapor)
- Multilayer adsorption (vapor)
- Capillary condensation (vapor-liquid)
- Non-saturated capillary flow (liquid)
- Saturated capillary flow (liquid)
- Capillary osmosis (liquid-solid)
- Electro-osmosis (liquid-solid)
- Streaming potential (liquid-solid)
- Diffusionphoresis (liquid solid)
- Evaporation (liquid-vapor)
- ...
- and most likely many others
Research Papers
Here are some of the research papers used for the compilation of the above information: