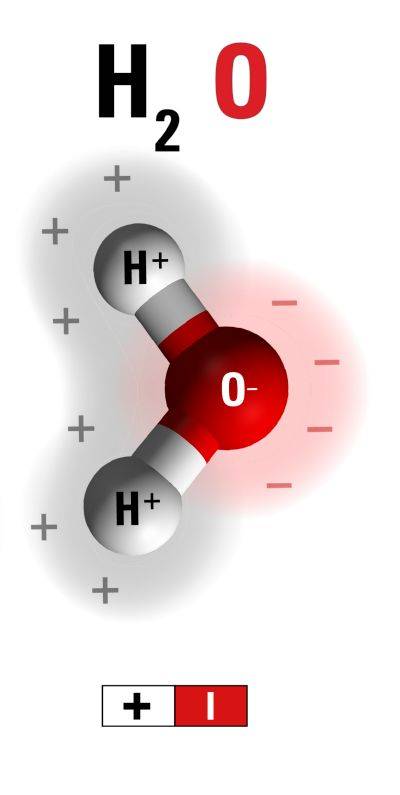
The Water Molecule
Water (H2O) consists of two positive hydrogen and one negative oxygen atom. This combination overall is neutral as the opposite charges (H2 and O) balance each other out.
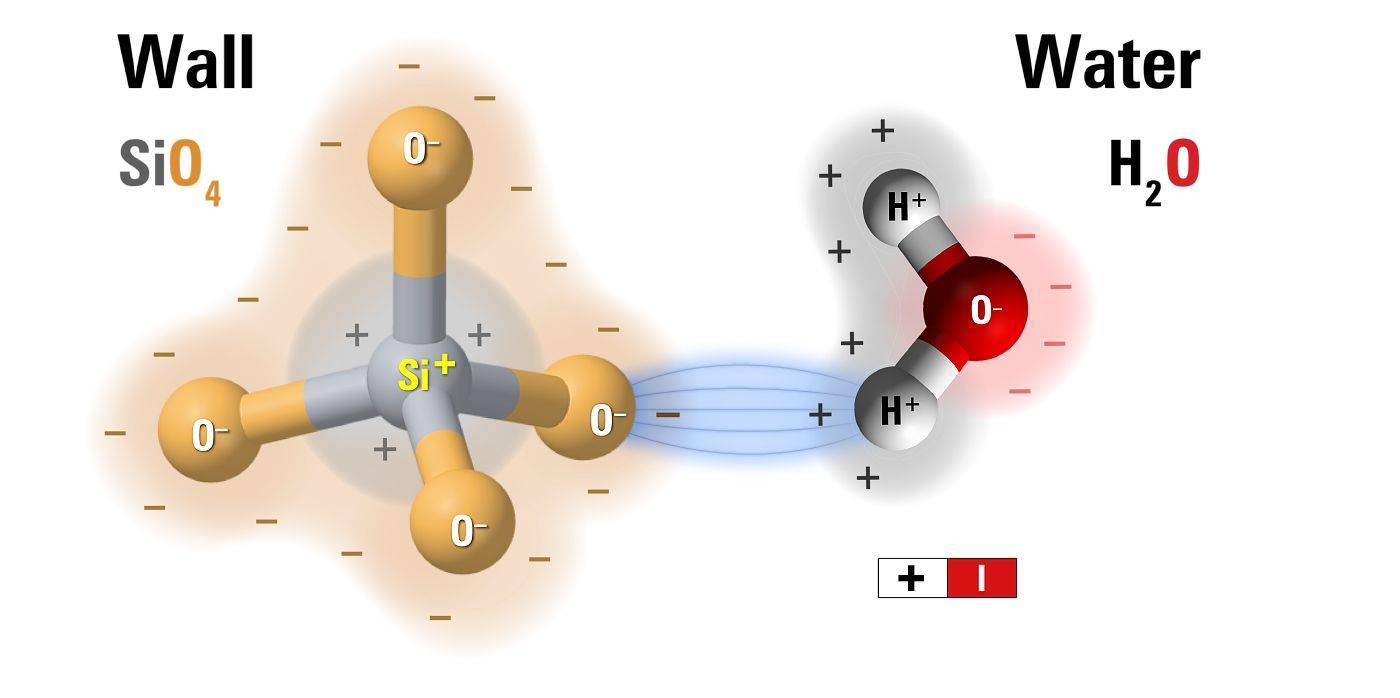
Silicate and water molecules
Capillary Wall - Water Interaction
The situation near a capillary surface changes significantly as capillaries carry a surface charge.
All building materials (brick, stone, mortar, glass, ceramics etc.) contain silica sand as their primary ingredient. The surface charge of silica is negative, hence capillary surfaces are negative. In contact with water they attract the opposite charges - positive H2 and salt ions - resulting in a minus-plus electrical charge distribution along the capillary surface (along zone 1).
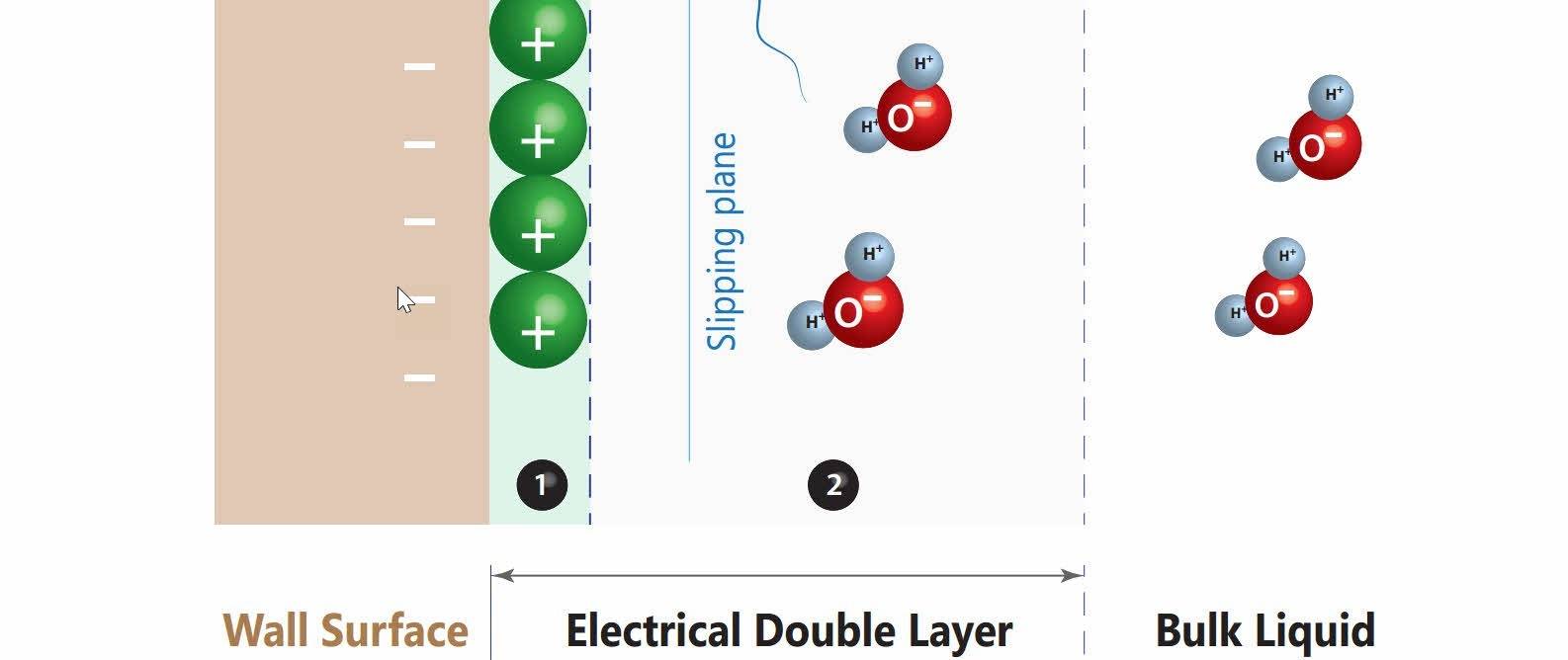
The electrical double layer
The attraction is so strong near the capillary surface (zone 1), that the water molecules are unable to move in relation to the capillaries - hence this is called the fixed layer (zone 1). The electrostatic attraction gradually weakens further away from the surface, allowing the water to flow (zone 2) - hence this is called the mobile or diffuse layer (zone 2).
These two sub-layers together form the electrical double layer (EDL) which is typically less than 10 nanometers wide.
The electrical double layer (EDL) is responsible for the bonding (adhesion) of water onto the capillary surfaces, which is electrostatic in nature. Capillary action, the up-down movement of water along a capillary surface, is the movement of the mobile layer (zone 2) against the fixed layer (zone 1) and the wall capillary.
Electrical double layers are formed in nature at the interface of any solid-liquid. The forces responsible for the attraction of opposite charges in the double layer are called electrokinetic forces - we are going to look at these next. These forces are very small, but on very short distances they are very strong, significantly stronger than gravity.
Summary
- The surface of most solid submerged in water acquires a negative surface charge, In combination with the positive charges from the water, this will form the electrical double layer (EDL).
- The electrostatic attraction of the oppositely charged molecules in the electrical double layer is responsible for the bonding of liquids to solid surfaces. (adhesion forces)
- Electrical double layers occur at the boundary of every solid-liquid.
- There are a lot of forces at play in the EDL area, discussed in the next section.