Short-range Forces Inside the Capillaries
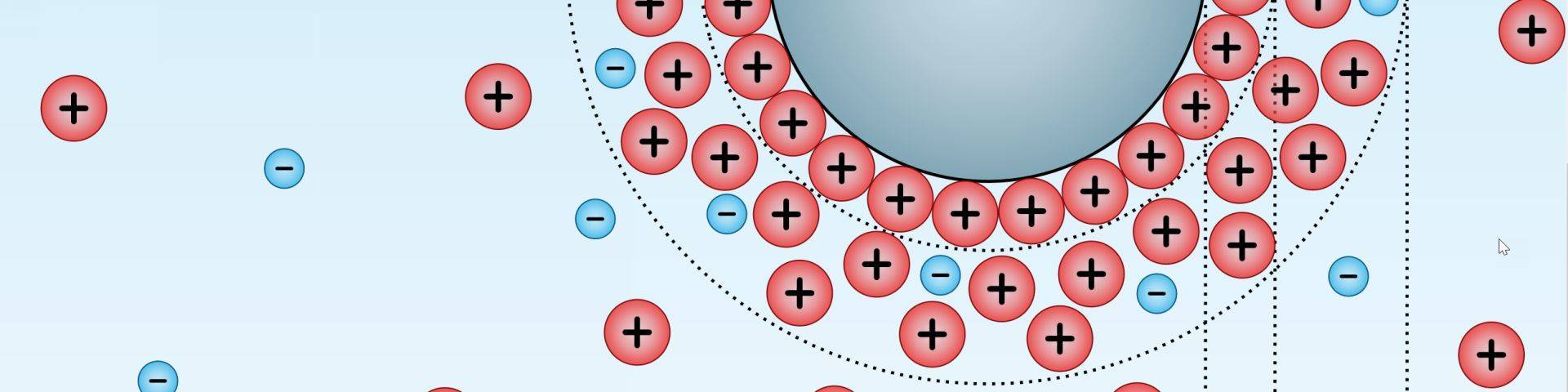
We discussed earlier that when a liquid comes into contact with a solid surface, a strong electrostatic attraction occurs between them. This attraction is due to a number of strong, short-distance molecular forces that exist inside the electrical double layer.
These forces are significantly stronger than gravity, which explains why liquids can rise against gravity. These are called elektrokinetic forces and the phenomena produced by them is known as elektrokinetic phenomena or effects. Electrokinetic refers to the fact that electrical effects create mechanical motion (electro = electrical, kinetic = motion).
Electrokinetic Phenomena
Electrokinetic phenomena describe the movement of fluids or solid particles under the influence of an electric field and vice-versa: electrical effects generated by moving liquid or solid particles as a result of pressure or concentration differences or gravity.
Electrokinetic phenomena encompass a large number of molecular phenomena, describing various combinations of movement and driving factors - whether a liquid is moving in confined spaces (e.g. water inside a capillary), or suspended solid particles are moving in liquids (e.g. diluted salt particles floating in water) etc. So there are quite a few available combinations.
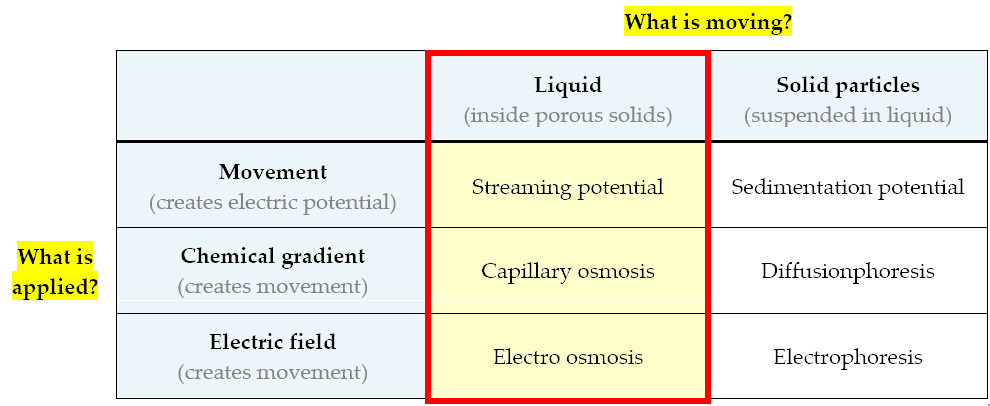
A quick summary of the main electrokinetic phenomena, grouped in a logical sequence, is shown in the table below:
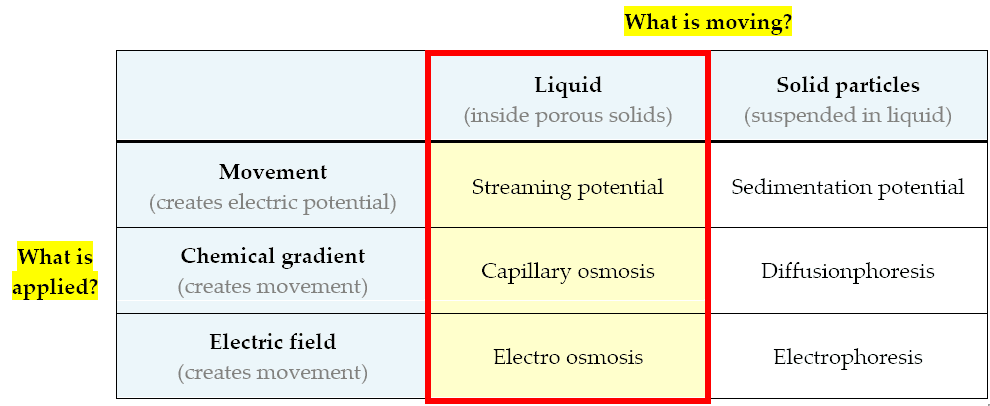
A summary of elektrokinetic phenomena
The detailed understanding of these phenomena is probably not important for a causal reader.
Summary
What is important to remember:
- There are a number of short-range molecular forces acting inside the capillaries (more exactly inside the thin electrical double layer) that determine and drive the movement of liquids inside capillaries.
- On short distances these forces are stronger than gravity, which explains why liquids can move up, against gravity, inside small capillaries.
- Not only one, but several of these forces play a significant role in capillary movement, as we are dealing with a complex system with at least 3 variables: charged capillaries interacting with a charged liquid (water being by-polar in nature) interacting with diluted salts carrying electrical charges.
- The exact interaction of this complex molecular system is still not fully understood, it is still under research.
For anyone interested, here is a brief explanation of the different elektrokinetic effects listed above:
Here are the phenomena related to the movement of liquids inside porous building materials:
- Capillary osmosis (or simply: osmosis) [concentration > movement]: the motion of a liquid in a porous material (osmotic flow) driven by differences in salinity (concentration differences)
- Electro-osmosis [electric potential > movement]: the motion of a liquid in a porous material under the influence of an electric field. It is the opposite of streaming potential.
- Streaming potential [movement > electric potential]: electrical potential generated by a fluid moving through a porous material. It is the opposite of electro-osmosis.
These are phenomena related to the movement of tiny solid particles floating inside a liquid:
- Diffusionphoresis [concentration > movement]: the motion of particles driven by differences in salinity (chemical concentration differences)
- Electrophoresis [electric potential > movement]: the motion of particles under the influence of an electric field.
- Sedimentation potential [movement > electric potential]: an electric potential generated by particles moving under the influence of gravity.